Opioid overdoses are becoming
INCREASINGLY
CHALLENGING
OVERDOSE HAS NEVER BEEN HIGHER.

Representative of respective lethal doses.
Opioid overdoses are becoming
INCREASINGLY
CHALLENGING
SYNTHETIC OPIOID
OVERDOSE HAS NEVER
BEEN HIGHER.

Representative of respective lethal doses.
FENTANYL:
100x
more potent
than morphine with a faster onset of action1,2
CARFENTANIL:
10,000x
more potent
than morphine—the most potent fentanyl analog in the United States3
85%
OF OPIOID OVERDOSE DEATHS
were due to synthetic opioids from April 2020 to April 20214
FENTANYL:
100x
more potent
than morphine with a faster onset of action1,2
CARFENTANIL:
10,000x
more potent
than morphine—the most potent fentanyl analog in the United States3
85%
OF OPIOID OVERDOSE DEATHS
were due to synthetic opioids from April 2020 to April 20214
THE NUMBER OF SEIZED COUNTERFEIT PILLS CONTAINING FENTANYL
DURING AN 8-WEEK PERIOD IN 2021 WAS ENOUGH TO POTENTIALLY KILL
>700,000 PEOPLE5
THE NUMBER OF SEIZED
COUNTERFEIT PILLS
CONTAINING FENTANYL
DURING AN 8-WEEK PERIOD IN
2021 WAS ENOUGH TO
POTENTIALLY KILL >700,000
PEOPLE5
GREATER ASSURANCE DURING AN OPIOID
OVERDOSE EMERGENCY RESCUE SITUATION
There is no way to know how much naloxone will be needed to restore normal respiration during an opioid overdose
emergency. When you consider all the uncertainties associated with an overdose—such as how much a patient has
taken or how long they haven’t been breathing—having a wider safety net could be beneficial.6
High-dose intramuscular naloxone administration could be the difference.
≈50%
OF OPIOID REVERSALS
are estimated to require more
than 1 dose of naloxone7
44%
4-mg INTRANASAL DOSE
of naloxone has only 44% of the
relative bioavailability* compared with
a 0.4-mg intramuscular injection8
GREATER ASSURANCE
DURING AN OPIOID
OVERDOSE
EMERGENCY RESCUE
SITUATION
There is no way to know how much
naloxone will be needed to restore
normal respiration during an opioid
overdose emergency. When you
consider all the uncertainties associated
with an overdose—such as how much a
patient has taken or how long they
haven’t been breathing—having a wider
safety net could be beneficial.6
High-dose intramuscular
naloxone administration
could be the difference.
≈50%
OF OPIOID REVERSALS
are estimated to require more than 1 dose
of naloxone7
44%
4-mg INTRANASAL DOSE
of naloxone has only 44% of the
relative bioavailability* compared
with a 0.4-mg intramuscular
injection7
EVEN WITH INTRAMUSCULAR ADMINISTRATION OF
NALOXONE, FENTANYL POTENCY DEMANDS GREATER
STRENGTH
As noted in a 2016 FDA Advisory Report, reducing opioid receptor occupancy to 50%
is estimated to be the threshold for successful overdose recovery6,9
Modeled simulation for rate of opioid receptor occupancy reduction of
fentanyl (50ng/mL) for 5-mg IM naloxone vs 2-mg IM naloxone10
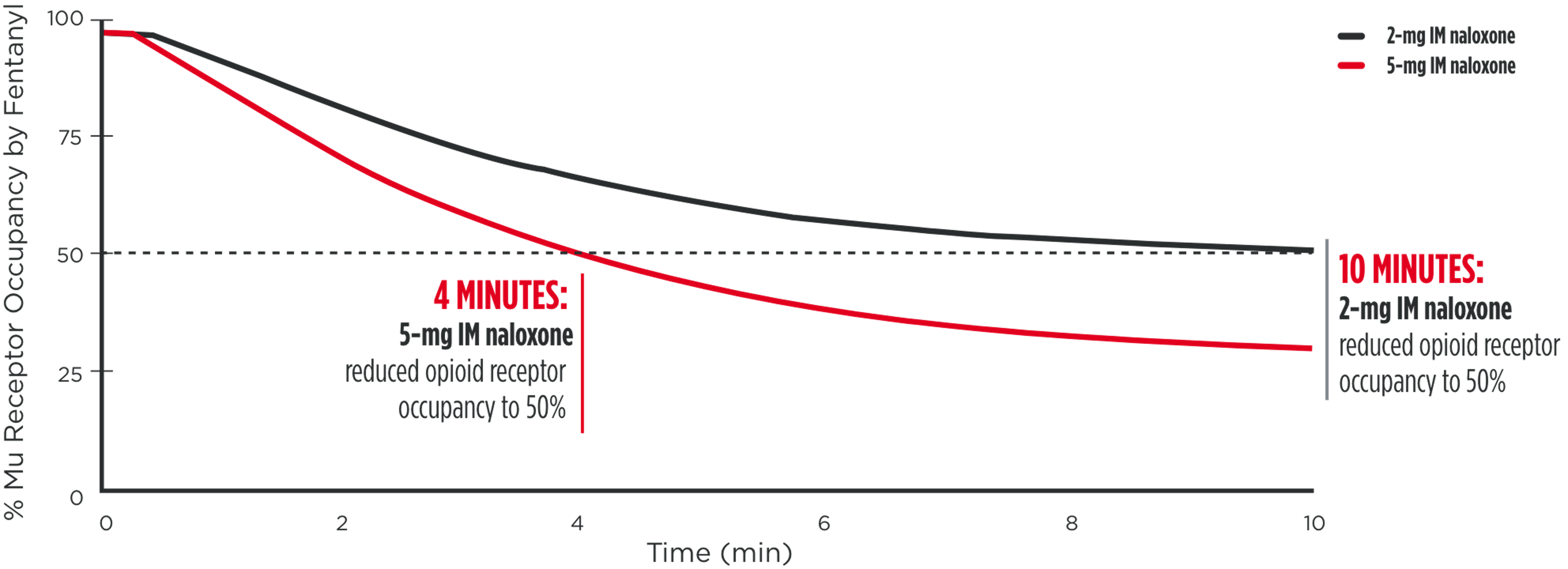
IM=intramuscular.
*Dose normalized.
EVEN WITH INTRAMUSCULAR ADMINISTRATION OF
NALOXONE, FENTANYL POTENCY DEMANDS GREATER STRENGTH
As noted in a 2016 FDA Advisory Report, reducing opioid receptor occupancy to 50%
is estimated to be the threshold for successful overdose recovery6,9
Modeled simulation for rate of opioid receptor
occupancy reduction of fentanyl (50ng/mL) for
5-mg IM naloxone vs 2-mg IM naloxone10
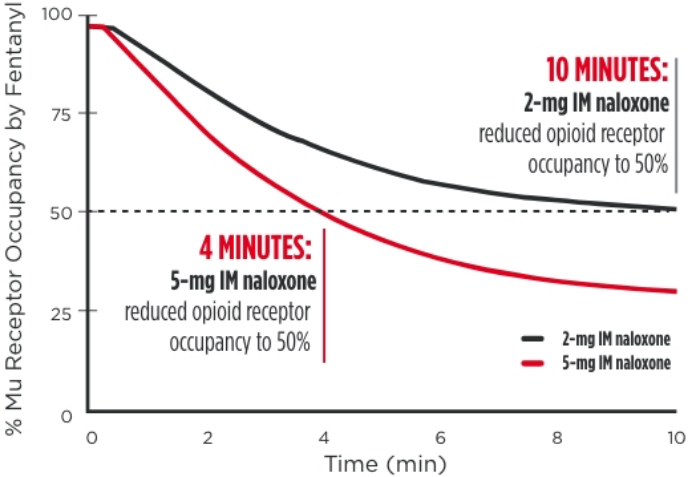
IM=intramuscular.
*Dose normalized.
“…if a patient is experiencing profound respiratory depression that could lead to death, the risks of precipitated withdrawal are outweighed by the need to restore respiration quickly before brain injury or death occur.”11
—FDA Spokesperson
FAST ABSORPTION WITH ZIMHI
HELPFUL TIPS FOR TALKING TO
PATIENTS ABOUT ZIMHI
ORDER ZIMHI
In an opioid overdose emergency,
ZIMHI MAY MAKE ALL
THE DIFFERENCE


In an opioid overdose emergency,
ZIMHI MAY
MAKE ALL THE
DIFFERENCE


In an opioid overdose emergency,
ZIMHI MAY
MAKE ALL THE
DIFFERENCE
INDICATION
ZIMHI is an opioid antagonist indicated for the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression in adult and pediatric patients. ZIMHI is intended for immediate administration as emergency therapy in settings where opioids may be present. ZIMHI is not a substitute for emergency medical care.
IMPORTANT SAFETY INFORMATION
As the duration of action of naloxone hydrochloride is shorter than many opioids, keep the patient under continued surveillance and administer repeated doses of naloxone using a new ZIMHI device, as necessary, while awaiting emergency medical assistance.
Reversal of respiratory depression caused by partial agonists or mixed agonists/antagonists, such as buprenorphine and pentazocine may be incomplete. Repeat doses of ZIMHI may be required.
Use in patients who are opioid dependent may precipitate opioid withdrawal. In neonates, opioid withdrawal may be life-threatening if not recognized and properly treated. Monitor for the development of signs and symptoms of opioid withdrawal.
Abrupt postoperative reversal of opioid depression may result in adverse cardiovascular (CV) effects. These events have primarily occurred in patients who had pre-existing CV disorders or received other drugs that may have similar adverse CV effects. Monitor these patients closely in an appropriate healthcare setting after use of naloxone hydrochloride.
After use, the ZIMHI needle is exposed until the safety guard is deployed. A needlestick injury could occur during use in emergency situations. In the event of accidental needlestick injury, medical attention should be sought.
The following adverse reactions were most commonly observed in ZIMHI clinical studies: dizziness, lightheadedness, and elevated bilirubin.
To report SUSPECTED ADVERSE REACTIONS, call 1-800-230-3935 or FDA at
1-800-FDA-1088 or www.fda.gov/medwatch
IMPORTANT SAFETY INFORMATION and INDICATION for ZIMHI™
As the duration of action of naloxone hydrochloride is shorter than many opioids, keep the patient under continued surveillance and administer repeated doses of naloxone using a new ZIMHI device, as necessary, while awaiting emergency medical assistance.
Reversal of respiratory depression caused by partial agonists or mixed agonists/antagonists, such as buprenorphine and pentazocine may be incomplete. Repeat doses of ZIMHI may be required.
IMPORTANT SAFETY INFORMATION and INDICATION for ZIMHI™



