INCREASINGLY
CHALLENGING

Representative of respective lethal doses.
INCREASINGLY
CHALLENGING

Representative of respective lethal doses.
FENTANYL:
100x
more potent
than morphine with a faster onset of action1,2
CARFENTANIL:
10,000x
more potent
than morphine—the most potent fentanyl analog in the United States3
85%
were due to synthetic opioids from April 2020 to April 20214
FENTANYL:
100x
more potent
than morphine with a faster
onset of action1,2
CARFENTANIL:
10,000x
more potent
than morphine—the most potent fentanyl analog in the United States3
85%
were due to synthetic opioids from April 2020 to April 20214
430% INCREASE
in seized fentanyl-laced counterfeit pills since 2019; 40% contained a potentially lethal dose5
IS YOUR CURRENT NALOXONE CHOICE ENOUGH TO
HANDLE THIS ESCALATING THREAT?
≈50%
OF OPIOID REVERSALS
are estimated to require more
than 1 dose of naloxone5
44%
4-mg INTRANASAL DOSE
of naloxone has only 44% of the
relative bioavailability* compared with
a 0.4-mg intramuscular injection7
- While 1 to 2 doses of intranasal naloxone may be required for a heroin overdose, 5 to 10 doses may be required for some fentanyl overdoses8
- Nasal cavity obstruction and congestion can impair absorption for intranasal delivery—one study demonstrated a 28% increased risk of opioid-induced respiratory depression in patients who have nasal obstructions (11.6% of patients; N=7234)9-11
IS YOUR CURRENT NALOXONE CHOICE ENOUGH TO HANDLE THIS ESCALATING THREAT?
≈50%
OF OPIOID REVERSALS
are estimated to require more than 1 dose
of naloxone6
44%
4-mg INTRANASAL DOSE
of naloxone has only 44% of the
relative bioavailability* compared
with a 0.4-mg intramuscular
injection7
- While 1 to 2 doses of intranasal naloxone may be required for a heroin overdose, 5 to 10 doses may be required for some fentanyl overdoses8
- Nasal cavity obstruction and congestion can impair absorption for intranasal delivery—one study demonstrated a 28% increased risk of opioid-induced respiratory depression in patients who have nasal obstructions (11.6% of patients; N=7234)9-11
EVEN WITH INTRAMUSCULAR ADMINISTRATION OF
NALOXONE, FENTANYL POTENCY DEMANDS GREATER STRENGTH
As noted in a 2016 FDA Advisory Report, reducing opioid receptor occupancy to 50%
is estimated to be the threshold for successful overdose recovery12,13
Modeled simulation for rate of opioid receptor occupancy reduction of
fentanyl (50ng/mL) for 5-mg IM naloxone vs 2-mg IM naloxone14
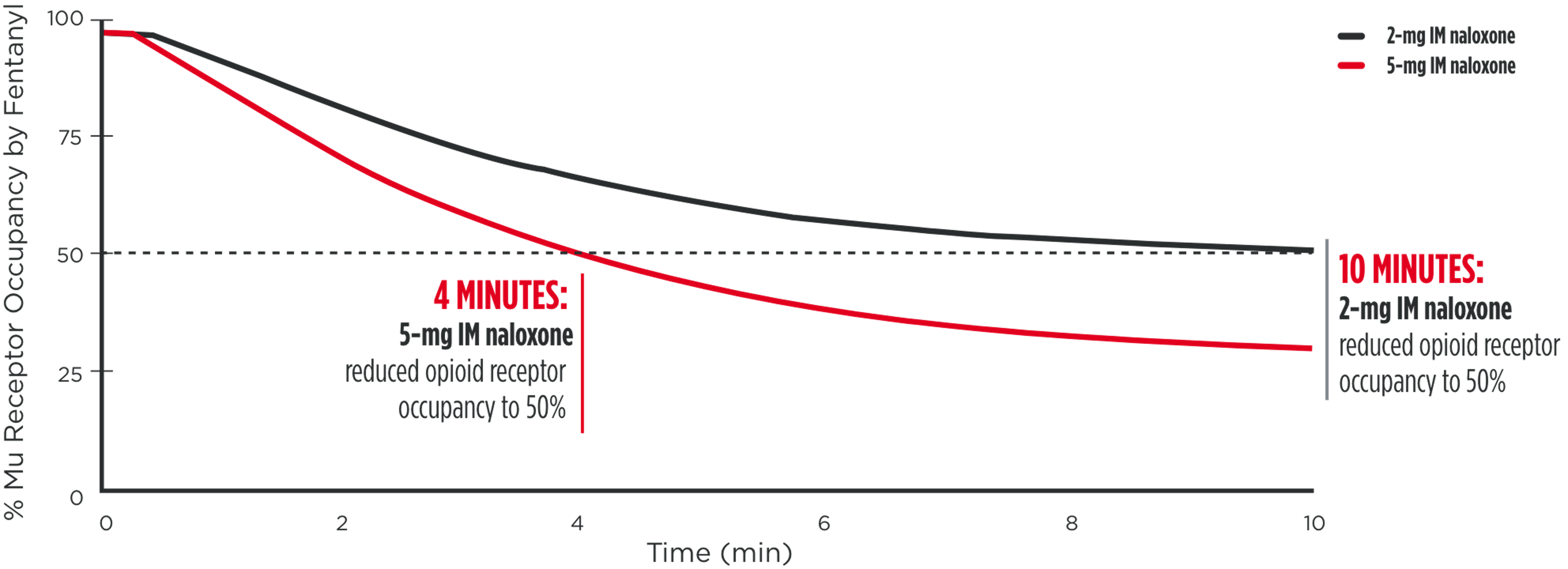
IM=intramuscular.
*Dose normalized.
EVEN WITH INTRAMUSCULAR ADMINISTRATION OF
NALOXONE, FENTANYL POTENCY DEMANDS GREATER STRENGTH
As noted in a 2016 FDA Advisory Report, reducing opioid receptor occupancy to 50%
is estimated to be the threshold for successful overdose recovery12,13
Modeled simulation for rate of opioid receptor
occupancy reduction of fentanyl (50ng/mL) for
5-mg IM naloxone vs 2-mg IM naloxone14
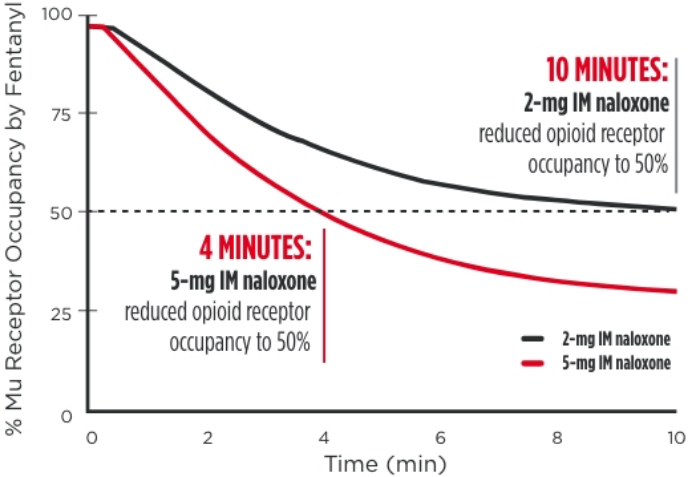
IM=intramuscular.
*Dose normalized.
“…if a patient is experiencing profound respiratory depression that could lead to death, the risks of precipitated withdrawal are outweighed by the need to restore respiration quickly before brain injury or death occur.”15
—FDA Spokesperson
FROM 2009 TO 2015, NEGATIVE OPIOID-OVERDOSE-RELATED ICU OUTCOMES
SIGNIFICANTLY INCREASED16
40%
INCREASE IN ICU ADMISSIONS
(44 per 10,000 admissions to 62 per 10,000; P<0.0001)
34%
INCREASE IN MORTALITY
(7.3% to 9.81%; P<0.0001)
58%
INCREASE IN COST PER ADMISSION
($58,517 to $92,408; P<0.0001)
FROM 2009 TO 2015, NEGATIVE OPIOID-OVERDOSE-RELATED ICU OUTCOMES
SIGNIFICANTLY INCREASED16
40%
INCREASE IN ICU ADMISSIONS
(44 per 10,000 admissions to 62 per 10,000; P<0.0001)
34%
INCREASE IN MORTALITY
(7.3% to 9.81%; P<0.0001)
58%
ADMISSION
($58,517 to $92,408; P<0.0001)
FAST NALOXONE ABSORPTION
WITH ZIMHI
VISIT THE ZIMHI TRAINING HUB
ORDER ZIMHI
In an opioid overdose emergency,
ZIMHI MAY MAKE ALL
THE DIFFERENCE
REQUEST A TRAINING DEVICE



In an opioid overdose emergency,
ZIMHI MAY
MAKE ALL THE
DIFFERENCE
REQUEST A TRAINING DEVICE



In an opioid overdose emergency,
ZIMHI MAY
MAKE ALL THE
DIFFERENCE
REQUEST A TRAINING DEVICE

References: 1. United States Drug Enforcement Administration. Fentanyl. Accessed October 28, 2020. https://www.dea.gov/factsheets/fentanyl 2. Stanley T. The fentanyl story. J Pain. 2014;15(12):1215-1226. doi:10.1016/j.jpain.2014.08.010 3. Centers for Disease Control and Prevention. Synthetic opioid overdose data. Updated March 25, 2021. Accessed November 30, 2021. https://www.cdc.gov/drugoverdose/deaths/synthetic/index.html 4. Centers for Disease Control and Prevention. Provisional drug overdose death counts. Updated October 13, 2021. Accessed December 9, 2021. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm 5. Department of Justice Announces DEA Seizures of Historic Amounts of Deadly Fentanyl-Laced Fake Pills in Public Safety Surge to Protect U.S. Communities. Department of Justice website. Published September 30, 2021. Accessed December 22, 2021. justice.gov/opa/pr/department-justice-announces-dea-seizures-historic-amounts-deadly-fentanyl-laced-fake-pills 6. Connecticut Department of Health. CT EMS SWORD: Statewide Opioid Reporting Directive Newsletter. CT DPH. Accessed October 20, 2021. https://portal.ct.gov/-/media/Departments-and-Agencies/DPH/dph/ems/pdf/SWORD/SWORD-newsletters/2021/SWORDSept2021NL_FINAL.pdf 7. Narcan. Prescribing Information. Adapt Pharma Operations Limited; Rev 08/2020. 8. Drug Strategies & Shatterproof. The fentanyl epidemic: state initiatives to reduce overdose deaths. 2019. 9. Dowley AC, Homer JJ. The effect of inferior turbinate hypertrophy on nasal spray distribution to the middle meatus. Clin Otolaryngol Allied Sci. 2001;26(6):488-490. doi:10.1046/j.1365-2273.2001.00509.x 10. Djupesland PG. Nasal drug delivery devices: characteristics and performance in a clinical perspective-a review. Drug Deliv Transl Res. 2013;3(1):42-62. doi:10.1007/s13346-012-0108-9 11. Weiner SG, Joyce AR, Thomson HN. The prevalence of nasal obstruction as a consideration in the treatment of opioid overdose. J Opioid Manag. 2017;13(2):69-76. doi:10.5055/jom.2017.0370 12. Melichar JK, Nutt DJ, Malizia AL. Naloxone displacement at opioid receptor sites measured in vivo in the human brain. Eur J Pharmacol. 2003:459(2-3);217-219. doi:10.1016/S0014-2999(02)02872-8 13. FDA Advisory Committee on the most appropriate dose or doses of naloxone to reverse the effects of life-threatening opioid overdose in the community settings. Adapt Pharma Operations Limited; 2016. 14. Moss RB, Pryor MM, Baillie R, et al. Higher naloxone dosing in a quantitative systems pharmacology model that predicts naloxone-fentanyl competition at the opioid mu receptor level. PLoS one. 2020;15(6):e0234683. doi:10.1371/journal.pone.0234683 15. Farah, T. How much naloxone is needed to reverse an opioid overdose? New high-dose treatments are raising questions. STAT. Published December 15, 2021. Accessed January 27, 2022. https://www.statnews.com/2021/12/15/naloxone-opioid-overdose-zimhi-kloxxado/ 16. Stevens JP, Wall MJ, Novack L, Marshall J, Hsu DJ, Howell MD. The critical care crisis of opioid overdoses in the United States. Ann Am Thorac Soc. 2017;14(12):1803-1809. doi:10.1513/AnnalsATS.201701-022OC
INDICATION
ZIMHI is a prescription medicine used in adults and children for the treatment of an opioid emergency, such as an overdose or a possible overdose with signs of breathing problems and severe sleepiness or not being able to respond. ZIMHI is to be given right away by a caregiver and does not take the place of emergency medical care. Get emergency medical help right away after the first dose of ZIMHI, even if the person wakes up.
IMPORTANT SAFETY INFORMATION
Do not use ZIMHI if you are allergic to naloxone hydrochloride or any of the ingredients in ZIMHI.
ZIMHI is used to temporarily reverse the effects of opioid medicines. The medicine in ZIMHI has no effect in people who are not taking opioid medicines.
Use ZIMHI right away if you or your caregiver think signs or symptoms of an opioid emergency are present, even if you are not sure, because an opioid emergency can cause severe injury or death.
Family members, caregivers, or other people who may have to use ZIMHI in an opioid emergency should know where ZIMHI is stored and how to give ZIMHI before an opioid emergency happens.
Get emergency medical help right away after using the first dose of ZIMHI. Rescue breathing or CPR may be given while waiting for emergency medical help.
The signs and symptoms of an opioid emergency can return within several minutes after ZIMHI is given. If this happens, give additional injections using a new ZIMHI prefilled syringe every 2 to 3 minutes and continue to closely watch the person until emergency help is received.
ZIMHI may cause serious side effects, including sudden opioid withdrawal symptoms, which may include: body aches, fever, sweating, runny nose, sneezing, goose bumps, yawning, weakness, shivering or trembling, nervousness, restlessness or irritability, diarrhea, nausea or vomiting, stomach cramping, increased blood pressure, or increased heart rate.
Other common side effects of ZIMHI include dizziness and injection site redness.
In infants under 4 weeks old who have been receiving opioids regularly, sudden opioid withdrawal may be life-threatening if not treated the right way. Signs and symptoms include: seizures, crying more than usual, and increased reflexes.
These are not all of the possible side effects of ZIMHI. Call your doctor for medical advice about side effects.
To report SUSPECTED ADVERSE REACTIONS, call 1-800-230-3935 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
IMPORTANT SAFETY INFORMATION and INDICATION for ZIMHI™
As the duration of action of naloxone hydrochloride is shorter than many opioids, keep the patient under continued surveillance and administer repeated doses of naloxone using a new ZIMHI device, as necessary, while awaiting emergency medical assistance.
Reversal of respiratory depression caused by partial agonists or mixed agonists/antagonists, such as buprenorphine and pentazocine may be incomplete. Repeat doses of ZIMHI may be required.
IMPORTANT SAFETY INFORMATION and INDICATION for ZIMHI™



